
Allergy Diagnostic Market Size to Hit Around USD13,193 Million by 2034
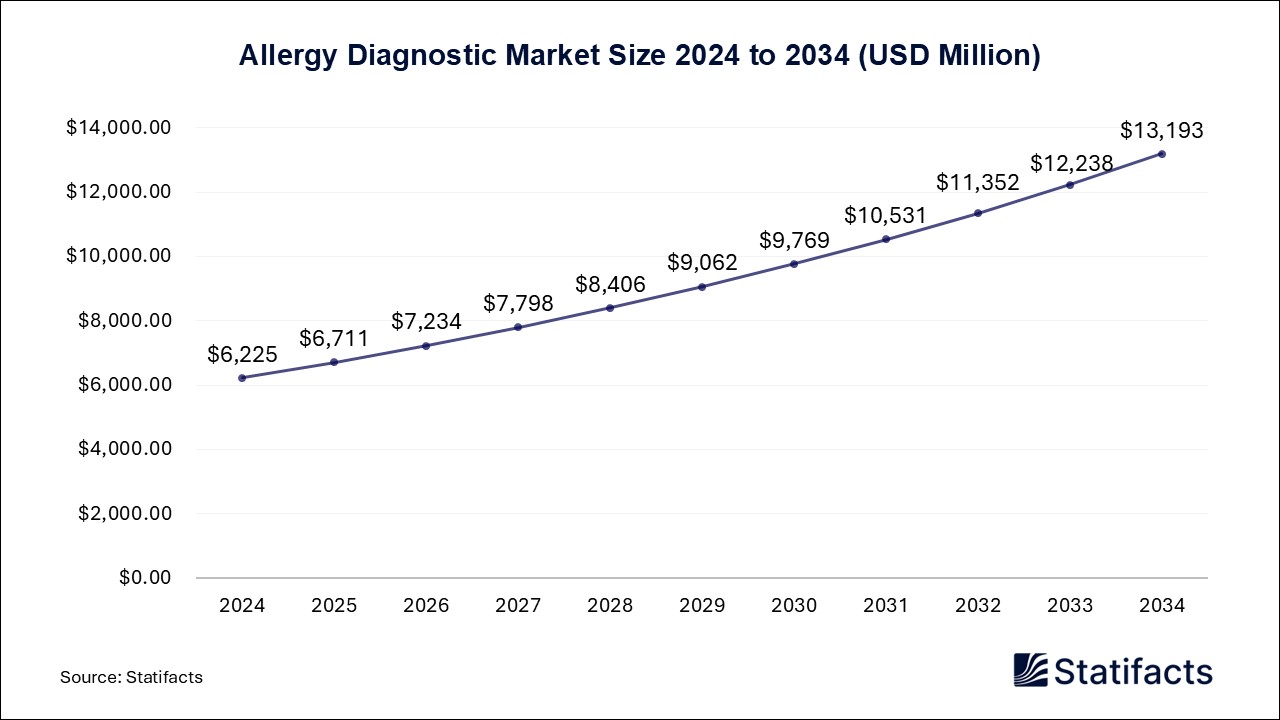
According to Statifacts, the global allergy diagnostic market size is calculated at USD 6,711 million in 2025 and is expected to reach around USD 13,193 million by 2033, growing at a CAGR of 7.8% from 2025 to 2034.
/EIN News/ -- Ottawa, Feb. 13, 2025 (GLOBE NEWSWIRE) -- The global allergy diagnostic market size was valued at USD 6,225 million in 2024, and is anticipated to reach around USD 13,193 million by 2034. It is expanding at a CAGR of 7.8% between 2025 and 2034,a study published by Statifacts a sister firm of Precedence Statistics.
Elevate your business strategy with market-driven insights—purchase the report today (Price USD1550) https://www.statifacts.com/order-report/7284
The increasing prevalence of allergic diseases due to rising pollution and the integration of technological advancements has driven the market growth.the increase in allergic diseases and related autoimmune disorders has skyrocketed due to excessive pollution, environmental changes, shifts in hygiene practices, and changing diets and lifestyles. These factors substantially contribute to the demand for allergy diagnostics in the market. The rise of the global industrial sector has led to higher temperatures and increased exposure to environmental pollutants. These ecological factors adversely affect human health by exposing them to disease risks, including the risk of allergic diseases like asthma, rhinitis, food allergies, and skin allergies. Apart from this, genetic factors also play a crucial role since individuals with pre-existing chronic diseases or who are socioeconomically disadvantaged are vulnerable and, therefore, easily afflicted by climate change. Urbanization, with its global temperatures, natural and anthropogenic pollutants, and hustle culture, with its distressed lifestyle, leads to dysregulation of the immune system, consequently fuelling the prevalence of allergic diseases, including food allergy, oral allergy syndrome, atopic dermatitis, allergic rhinitis, allergic conjunctivitis, allergic asthma, eosinophilic esophagitis, drug allergy, and venom allergy. Recent advancements in diagnostic production technologies, particularly molecular diagnostics and component-resolved diagnostics, are enhancing both the quality and accessibility of diagnosis and treatment. These innovations are paving the way for the creation of increasingly specialized and targeted tests tailored to meet specific allergic components.
Additionally, collaborations among research institutions and diagnostics companies are playing a crucial role in spurring innovation and extending the market reach for diagnostic tools. Public awareness and personalized medicines help to identify allergies and their treatment plans. Early diagnosis of allergies and access to health care are ultimately enhancing and improving the allergy diagnostic market.
The integration of artificial intelligence (AI) into the allergy diagnostic market is proving transformative. Advanced AI algorithms enhance patient outcomes by shifting large amounts of data from different sources such as electronic health records, lab results, and result testing—this is a remarkable method that helps in identifying and predicting a patient’s allergy and its triggering reason. Moreover, AI's cutting-edge capabilities can process extensive data sets, enabling it to understand and gather records of allergic diseases and immune system responses and optimize them to develop accurate, effective, and tailored treatments. As a result, researchers are empowered to design diagnostic tests that are not only more effective but also safer, thereby reducing the necessity for expensive and time-intensive experimental trials. AI can also detect allergens by utilizing AI-assisted imaging techniques, including hyperspectral imaging, wherein the food samples can be analyzed with microscopic precision. This AI analysis is essential for the development of effective diagnostics, ultimately transforming patient care and treatment tests.
Allergy Diagnostic Market Report Scope
| Report Attribute | Details |
| Market size value in 2025 | USD 6.711 Million |
| Revenue forecast in 2034 | USD 13,193 Million |
| Growth rate | CAGR of 7.8 % from 2025 to 2034 |
| Actual data | 2018 - 2024 |
| Forecast period | 2025 - 2034 |
| Quantitative units | Revenue in USD million/billion, and CAGR from 2025 to 2034 |
| Report coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments covered | Product, Allergen , Test, end use, region |
| Regional scope | North America, Europe, Asia Pacific, Latin America, MEA |
| Country scope | U.S., Canada, Mexico, Germany, UK, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, South Korea, Australia, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, and Kuwait |
| Key companies profiled | Hitachi Chemical Co. Ltd; Thermo Fisher Scientific Inc.; Siemens Healthineers.; Danaher Corporation; HOB Biotech Group Co; bioMérieux; Hycor Biomedical Inc.; Stallergenes Greer; R-Biopharm AG; Lincoln Diagnostics Inc. |
Elevate your business strategy with market-driven insights—purchase the report today (Price USD1550) https://www.statifacts.com/order-report/7284
What Types of Tests Do Doctors Use to Diagnose Allergies ?
Skin Prick Test (SPT)
Skin testing can confirm many common types of allergies. In some cases, skin tests can be the most accurate and least expensive way to confirm allergens. For prick/scratch testing, the doctor or nurse places a small drop of the possible allergen on the skin. They will then lightly prick or scratch your skin with a needle through the drop. If you are sensitive to the substance, you will develop redness, swelling and itching at the test site within 15 minutes. You may also see a “wheal,” or raised, round area, that looks like a hive. Usually, the larger the wheal, the more likely you are to be allergic to the allergen.
It is important to know:
- A positive skin test result does not by itself diagnose an allergy.
- A positive skin test does not predict the severity of an allergic reaction.
-
A negative skin test usually means you are not allergic.
Intradermal Skin Test
In intradermal (under the skin) testing, the doctor or nurse injects a tiny amount of allergen into the outer layer of skin. The doctor checks your skin after a set amount of time for results, like with the skin prick test. Doctors may use this test if the skin prick test results are negative but they still suspect you have allergies. A doctor may use this test for diagnosing drug or venom allergy. At this time, there are very few indications for intradermal skin testing for food allergy.
Blood Tests (Specific IgE)
If you have a skin condition or are taking medicine that interferes with skin testing, allergen blood tests may be used. They may also be used for children who may not tolerate skin testing. Your doctor will take a blood sample and send it to a laboratory. The lab adds the allergen to your blood sample and then measures the amount of antibodies your blood produces to attack the allergens. This test is called Specific IgE (sIgE) Blood Testing (previously and commonly referred to as RAST or ImmunoCAP testing). This test is a not a good screening test due to the high rates of false positive results. There is no test that can determine how severe an allergy is for someone.
Physician-Supervised Challenge Tests
In your doctor’s office, you inhale or take a tiny amount of an allergen by mouth. This test is usually done with possible medication or food allergies. A physician, usually an allergist, should supervise this test due to the risk of anaphylaxis, a severe life-threatening reaction.
Patch Test
This test determines what allergen may be causing contact dermatitis. Your doctor will place a small amount of a possible allergen on your skin, cover it with a bandage and check your reaction after 48 to 96 hours. If you are allergic to the substance, you should develop a local rash.
Allergy Diagnostic Market Trends
- Component-Resolved Diagnostics (CRD): Component-resolved diagnostics represent a significant advancement in allergy testing by going beyond merely identifying general allergens. Instead, CRD specifically detects the individual allergenic molecules responsible for triggering allergic reactions. This level of precision facilitates more accurate diagnoses, enhances risk management, and enables the development of tailored treatment plans for patients. Utilizing these cutting-edge technologies, healthcare providers can test for multiple allergens simultaneously from a single sample, thereby streamlining the testing process and ensuring comprehensive evaluation.
- Point of Care Testing (POCT): Point of care testing is rapidly gaining traction in the medical field due to its ability to deliver quick and reliable results, often in a matter of minutes. These tests can be conducted in various settings, including doctor's offices, clinics, and even the comfort of one's home. This immediacy is particularly beneficial for diagnosing food allergies and other conditions where timely intervention is essential. Recent advancements in microfluidics, along with lab-on-a-chip technologies, are paving the way for the creation of portable and user-friendly POCT devices that simplify allergy testing for patients and practitioners alike.
- Personalized Medicines: The shift towards personalized medicine is becoming increasingly prominent as it assists in harnessing data from multiple sources to provide a more nuanced understanding of an individual's allergies. This holistic approach helps inform individualized treatment strategies and management plans. There's a notable rise in the exploration of biomarkers and artificial intelligence to assess the likelihood of developing allergies or experiencing severe allergic reactions. With this predictive capability, healthcare providers can implement proactive interventions and preventive strategies, potentially reducing the risk of acute allergic responses.
- Public Awareness - As food allergies continue to rise in prevalence, public awareness of their seriousness is also increasing. This heightened attention is driving the demand for accurate and dependable food allergy testing methods. Innovations in technology are making it possible to detect even trace amounts of food allergens, significantly enhancing food safety standards and assisting individuals in effectively managing their allergies. Such developments are crucial for educating the public and ensuring that those with food allergies can navigate their environments safely.
Buy this Databook (Price USD1550) https://www.statifacts.com/order-report/7284
Allergy Diagnostic Market Segment Insights
Type Insights
The therapeutics segment emerged as the predominant revenue generator in the allergy diagnostic market, primarily due to its concentrated efforts in managing and alleviating allergic conditions. Therapeutic interventions are designed to effectively relieve the symptoms associated with allergies, addressing the fundamental issues that patients face. Among these interventions, epinephrine has established itself as the leading product, renowned for its quick and potent action in counteracting severe allergic reactions, such as anaphylaxis. Its ability to act rapidly makes it indispensable for individuals with life-threatening allergies.
Conversely, the diagnostic segment is projected to experience the fastest growth within the allergy diagnostic landscape. This growth is driven by an increasing need for accurate and timely identification of allergic conditions. Diagnostic tools play a crucial role in assessing sensitivities to specific allergens, which enables healthcare providers to implement targeted and effective therapeutic strategies.
Allergen Type Insights
When considering the types of allergens, the inhaled segment commanded the largest share of revenue within the allergy diagnostic market. This trend is closely tied to the rising prevalence of respiratory allergies, a phenomenon exacerbated by industrialization and heightened air pollution levels. The escalating rates of allergic rhinitis, characterized by symptoms such as sneezing and nasal congestion, are expected to create substantial opportunities for growth in the market's future.
In addition, drug allergies are anticipated to witness even swifter growth in the allergy diagnostic sector. These allergies can lead to severe reactions, including toxic epidermal necrolysis and Stevens-Johnson syndrome, particularly in vulnerable populations such as children. Common culprits for drug allergies include chemotherapy agents, sulfonamide medications, and various antiepileptic drugs. Reactions to these allergens can manifest as conditions like contact dermatitis, a painful inflammation of the skin.
Test Type Insights
In the realm of diagnostic methodologies, the in vitro test segment secured the highest revenue within the allergy diagnostic market. These tests are specifically employed to detect allergen-specific immunoglobulin E (IgE) in serum samples. The measurement of serum IgE levels is particularly valuable, as it boasts a high positive predictive value and superior specificity for identifying sensitization to aeroallergens, such as pollen and insect bites. Such characteristics are likely to enhance the penetration of allergy diagnostics in the market throughout the forecast period.
On the other hand, the in vivo test segment is expected to experience significant growth, fueled by an increasing adoption rate of various in vivo testing methods. These tests are celebrated for their convenience, cost-effectiveness, safety, and reliability compared to other diagnostic options. The rising demand for rapid, straightforward, and efficient allergy testing will drive the growth of in vivo diagnostic methodologies.
Regional Insights
Regionally, North America stands out as the dominant market for allergy diagnostics, accounting for the largest share of revenue. This is attributable to a combination of factors, including a sharp rise in allergy incidences and supportive government initiatives aimed at improving healthcare outcomes. As a developed region, North America has a high level of healthcare expenditure and a robust understanding of the significant potential associated with allergy medications.
In contrast, the Asia Pacific market is predicted to grow at a robust rate within the allergy diagnostic sector. This anticipated growth can be linked to several factors, including ongoing healthcare reforms and the expansion of healthcare infrastructure across the region. Additional contributors to this positive trend include a burgeoning population, a rising incidence of allergies, and a growing number of local companies entering the market, all of which are poised to bolster growth in the allergy diagnostic arena.
You can place an order or ask any questions, please feel free to contact us at sales@statifacts.com
Recent Developments
- In May 2024, ArtiQ.Spiro AI solution, an AI-based software by ArtiQ, showed 88% diagnostic accuracy and improved primary care in their study.
- In May 2023, Metropolis Healthcare Limited, India's leading diagnostic service provider, announced the launch of an AI-powered Allergy Component Testing platform, which was based on Component Resolved Diagnostics (CRD) and will support India's population in detecting various forms of allergies.
Allergy Diagnostic Market Top Key Companies:
- Hitachi Chemical Co. Ltd
- Thermo Fisher Scientific Inc.
- Siemens Healthineers
- Danaher Corporation
- HOB Biotech Group Co
- bioMérieux
- Hycor Biomedical Inc.
- Stallergenes Greer
- R-Biopharm AG
- Lincoln Diagnostics Inc.
Allergy Diagnostic Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2019 to 2034. For this study, Statifacts has segmented the global Allergy Diagnostic Market
By Product Type
- Fungal Allergy Diagnostic
- Neutral Lactase Enzymes
By Allergen Type
- Inhaled Allergens
- Food Allergens
- Drug Allergens
- Others
By Test Type
- In-vivo Allergy Tests
- In-vitro Allergy Tests
By End User
- Diagnostic Laboratories
- Hospitals
- Academic Research Institutes
- Others
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East Africa
Elevate your business strategy with market-driven insights—purchase the report today (Price USD1550) https://www.statifacts.com/order-report/7284
Browse More Research Reports ;
- U.S. Tuberculosis Diagnostics Market : The U.S. tuberculosis diagnostics market size accounted for USD 594.53 million in 2024 and is expected to exceed around USD 1,025.21 million by 2034, growing at a CAGR of 5.6% from 2024 to 2034.
- U.S. Ovarian Cancer Diagnostics Market: The U.S. ovarian cancer diagnostics market size was estimated at USD 2,109 million in 2024 and is expected to be worth around USD 3,092 million by 2034, growing at a compound annual growth rate (CAGR) of 3.9% over the forecast period 2025 to 2034.
- U.S. Oncology Molecular Diagnostic Market : The U.S. oncology molecular diagnostic market size accounted for USD 792 million in 2024 and is expected to exceed around USD 2,527 million by 2034, growing at a CAGR of 12.3% from 2025 to 2034.
- U.S. In Vitro Diagnostics Contract Manufacturing Market: The U.S. in vitro diagnostics contract manufacturing market size was valued at USD 8.19 billion in 2024 and is expected to be worth around USD 21.44 billion by 2034, growing at a compound annual growth rate (CAGR) of 10.1% during the forecast period 2025 to 2034.
- U.S. Autoimmune Disease Diagnostics Market : The U.S. autoimmune disease diagnostics market size was estimated at USD 2,219 million in 2024 and is projected to hit around USD 3,719 million by 2034, growing at a CAGR of 5.3% during the forecast period from 2025 to 2034.
- U.S. Lung Cancer Diagnostics Market : The U.S. lung cancer diagnostics market size was estimated at USD 4,779 million in 2024 and is projected to be worth around USD 10,414 million by 2034, growing at a CAGR of 8.1% from 2025 to 2034.
- U.S. Cancer Diagnostics Market : The U.S. cancer diagnostics market size accounted for USD 44,171 million in 2024 and is predicted to touch around USD 82,915 million by 2034, growing at a CAGR of 6.5% from 2025 to 2034.
- U.S. Breast Cancer Diagnostics Market : The U.S. breast cancer diagnostics market size is calculated at USD 7,809 million in 2024 and is predicted to attain around USD 16,703 million by 2034, expanding at a CAGR of 7.9% from 2025 to 2034.
-
U.S. Next Generation Cancer Diagnostics Market : The U.S. next generation cancer diagnostics market size was estimated at USD 5,119 million in 2024 and is expected to be worth around USD 11,052 million by 2034, growing at a compound annual growth rate (CAGR) of 8% over the forecast period 2025 to 2034.
You can place an order or ask any questions, please feel free to contact us at sales@statifacts.com
Statifacts offers subscription services for data and analytics insights. This page provides options to explore and purchase a subscription tailored to your needs, granting access to valuable statistical resources and tools. Access here - https://www.statifacts.com/get-a-subscription
Contact US
- Ballindamm 22, 20095 Hamburg, Germany
- Email: sales@statifacts.com
- Web: https://www.statifacts.com/
-
Europe : +44 7383 092 044
About US
Statifacts is a leading provider of comprehensive market research and analytics services, offering over 1,000,000 market and custoer data sets across various industries. Their platform enables businesses to make informed strategic decisions by providing full access to statistics, downloadable in formats such as XLS, PDF, and PNG.
For Latest Update Follow Us:
Statifacts | Precedence Research| Towards Healthcare

Distribution channels: Business & Economy ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

